Describe the electrodes in this iron-copper galvanic cell – Electrodes in iron-copper galvanic cells play a crucial role in the electrochemical reactions that generate electrical energy. This article delves into the intricacies of these electrodes, exploring their types, materials, dimensions, surface area, placement, and connections. By unraveling the characteristics and significance of these components, we gain a deeper understanding of the fundamental principles governing galvanic cells.
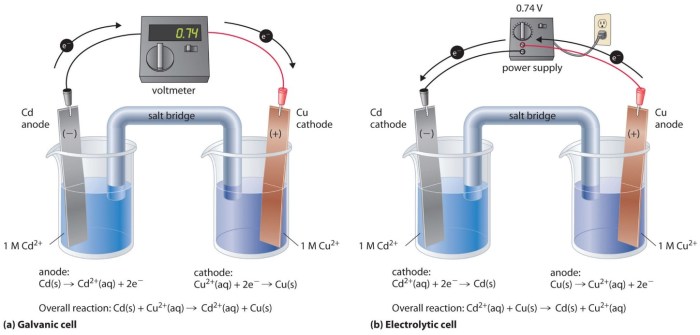
The two electrodes in an iron-copper galvanic cell are the anode and the cathode. The anode is the electrode where oxidation occurs, while the cathode is where reduction occurs. In this specific cell, iron serves as the anode, and copper serves as the cathode.
Electrodes in Iron-Copper Galvanic Cell: Describe The Electrodes In This Iron-copper Galvanic Cell
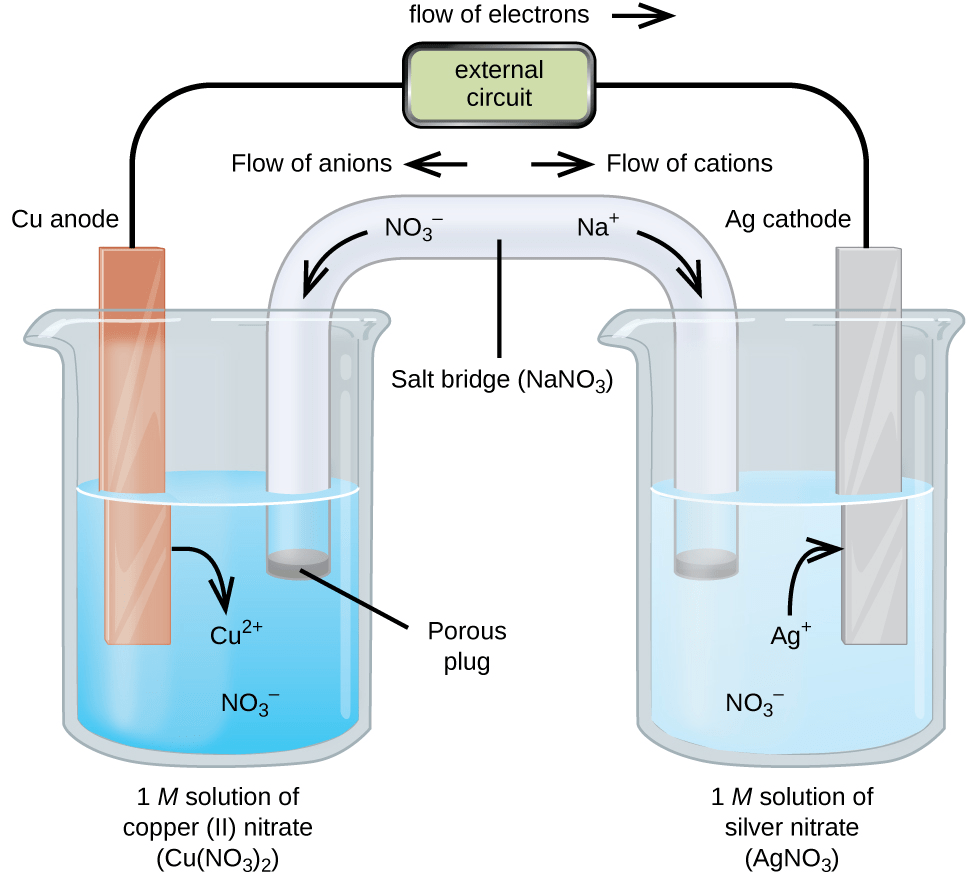
Galvanic cells rely on two electrodes, each serving a distinct role in the electrochemical process. The anode undergoes oxidation, losing electrons, while the cathode undergoes reduction, gaining electrons.
Electrode Types
In the iron-copper galvanic cell, the anode is made of iron (Fe), and the cathode is made of copper (Cu). The anode undergoes oxidation, releasing iron ions (Fe2+) into the solution, while the cathode undergoes reduction, depositing copper atoms (Cu) onto its surface.
Electrode Materials
Iron is chosen for the anode due to its high oxidation potential, which makes it prone to losing electrons. Copper is chosen for the cathode because it has a low reduction potential, making it receptive to gaining electrons.
Electrode Dimensions and Shape
The dimensions and shape of the electrodes affect the cell’s performance. Larger electrodes provide a greater surface area for electrochemical reactions, increasing the current flow. The shape of the electrodes can also influence the current distribution, affecting the cell’s efficiency.
Electrode Surface Area
The surface area of the electrodes is crucial for the cell’s performance. A larger surface area allows for more active sites for electrochemical reactions, increasing the current flow and the rate of the reaction.
Electrode Placement
The electrodes are positioned within the cell to maximize the electrochemical reactions. The anode and cathode are placed in separate compartments to prevent direct contact between the reactants and products. This separation allows for the buildup of ions in the solution, maintaining a concentration gradient that drives the electrochemical process.
Electrode Connections, Describe the electrodes in this iron-copper galvanic cell
The electrodes are connected to an external circuit, completing the electrical pathway for the flow of electrons. The external circuit provides a load for the cell, allowing the electrons to flow from the anode to the cathode, generating an electric current.
FAQ Section
What is the difference between an anode and a cathode?
In a galvanic cell, the anode is the electrode where oxidation occurs, while the cathode is where reduction occurs.
Why are iron and copper used as the electrodes in this cell?
Iron and copper are chosen because they have different reduction potentials, which allows for a spontaneous electrochemical reaction to occur.