Electrons protons and neutrons worksheet – Embark on a captivating exploration of the fundamental particles that constitute the very essence of matter with our comprehensive Electrons, Protons, and Neutrons Worksheet. This meticulously crafted resource delves into the intriguing properties, interactions, and applications of these subatomic building blocks, providing a profound understanding of the microscopic world that governs our existence.
Through engaging activities, comparative tables, and insightful quotes from renowned scientists, this worksheet illuminates the significance of electrons, protons, and neutrons in shaping the chemical reactions, atomic structures, and technological advancements that have transformed our understanding of the universe.
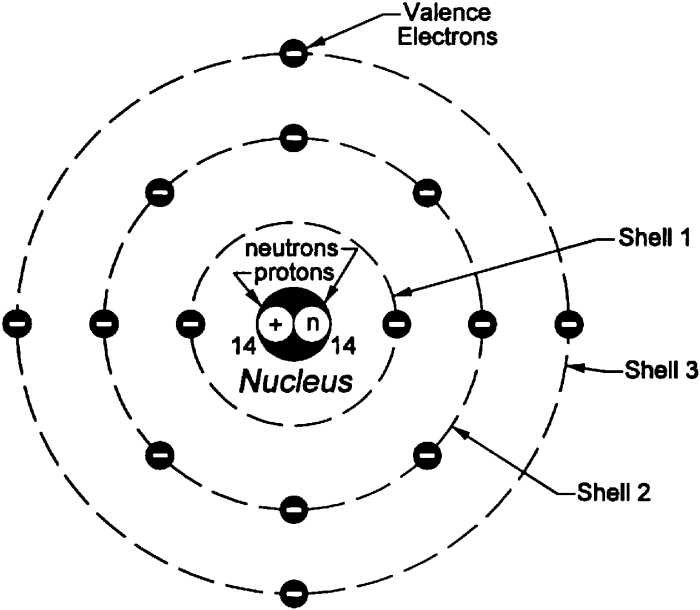
Properties of Electrons, Protons, and Neutrons
Fundamental particles known as electrons, protons, and neutrons are the building blocks of atoms. Each particle possesses unique properties that contribute to the structure and behavior of matter.
Electrons, Electrons protons and neutrons worksheet
- Charge: Negative (-1e)
- Mass: Negligible (1/1836 of a proton)
- Location: Orbit around the nucleus in electron shells
Protons
- Charge: Positive (+1e)
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Reside in the nucleus of the atom
Neutrons
- Charge: Neutral (0e)
- Mass: Slightly greater than a proton (1.0087 amu)
- Location: Reside in the nucleus of the atom alongside protons
Interactions between Electrons, Protons, and Neutrons
The interactions between these particles play a crucial role in determining the behavior and stability of atoms.
Electrostatic Forces
Electrons and protons possess opposite charges, resulting in an electrostatic attraction between them. This force holds the electrons in orbit around the nucleus.
Strong Nuclear Force
The strong nuclear force is a powerful attraction that binds protons and neutrons together in the nucleus. This force overcomes the electrostatic repulsion between protons.
Contributions to Atomic Structure
The interplay of these forces determines the structure and stability of atoms. The number of electrons and protons in an atom determines its chemical properties, while the number of neutrons affects the stability of its nucleus.
Role of Electrons, Protons, and Neutrons in Chemical Reactions: Electrons Protons And Neutrons Worksheet
These particles play a fundamental role in chemical reactions, which involve the rearrangement of atoms and molecules.
Electron Involvement in Chemical Bonding
Electrons are the primary participants in chemical bonding. They are involved in covalent bonding, where electrons are shared between atoms, and ionic bonding, where electrons are transferred between atoms.
Protons and Chemical Properties
The number of protons in an atom’s nucleus determines its atomic number, which defines the element. The arrangement of electrons around the protons influences the chemical properties of the element.
Neutron Effects on Stability
The number of neutrons in an atom’s nucleus affects its stability. Isotopes are atoms of the same element with varying numbers of neutrons. The stability of isotopes depends on the neutron-to-proton ratio.
Applications of Understanding Electrons, Protons, and Neutrons
Knowledge of these particles has numerous applications in various fields.
Medical Applications
Understanding the behavior of electrons and protons is essential in radiation therapy for treating cancer.
Energy Applications
Nuclear reactions involving protons and neutrons are harnessed in nuclear power plants to generate electricity.
Materials Science
The properties of electrons and protons influence the electrical and magnetic properties of materials, which are crucial in the development of new technologies.
Advancements in Technology
Understanding these particles has led to the development of advanced technologies such as particle accelerators, electron microscopes, and nuclear medicine.
Worksheet Activities
The following activities provide opportunities to enhance understanding of electrons, protons, and neutrons.
Worksheet Exercises
- Identify the charge, mass, and location of electrons, protons, and neutrons.
- Compare the properties of these particles in a table.
- Explain the electrostatic forces and strong nuclear force between these particles.
Quotes from Scientists
“The electron is the most fundamental unit of matter.”
Richard Feynman
“The proton is the cornerstone of the nucleus.”
Ernest Rutherford
“The neutron is a mystery wrapped in an enigma.”
Niels Bohr
General Inquiries
What is the primary function of electrons?
Electrons play a crucial role in chemical bonding, determining the reactivity and properties of various elements.
How do protons contribute to the stability of atoms?
Protons, along with neutrons, form the nucleus of an atom, providing stability and defining its atomic number.
What is the significance of understanding the interactions between electrons, protons, and neutrons?
Comprehending these interactions is essential for unraveling the behavior of atoms, molecules, and the chemical reactions that shape our world.